
EXCEPTION OF CHROMIUM ELECTRON CONFIGURATION FULL
The important thing to note from these two examples is that there is no special added stability form merely having a half full electron shell (i.e., something is not magically more stable because of having a half filled subshell). Knowing this, it is energetically more favorable to have $4s^1$ $3d^9$ because the higher energy orbital only has one unpaired electrons and the lower energy orbitals have paired ones.
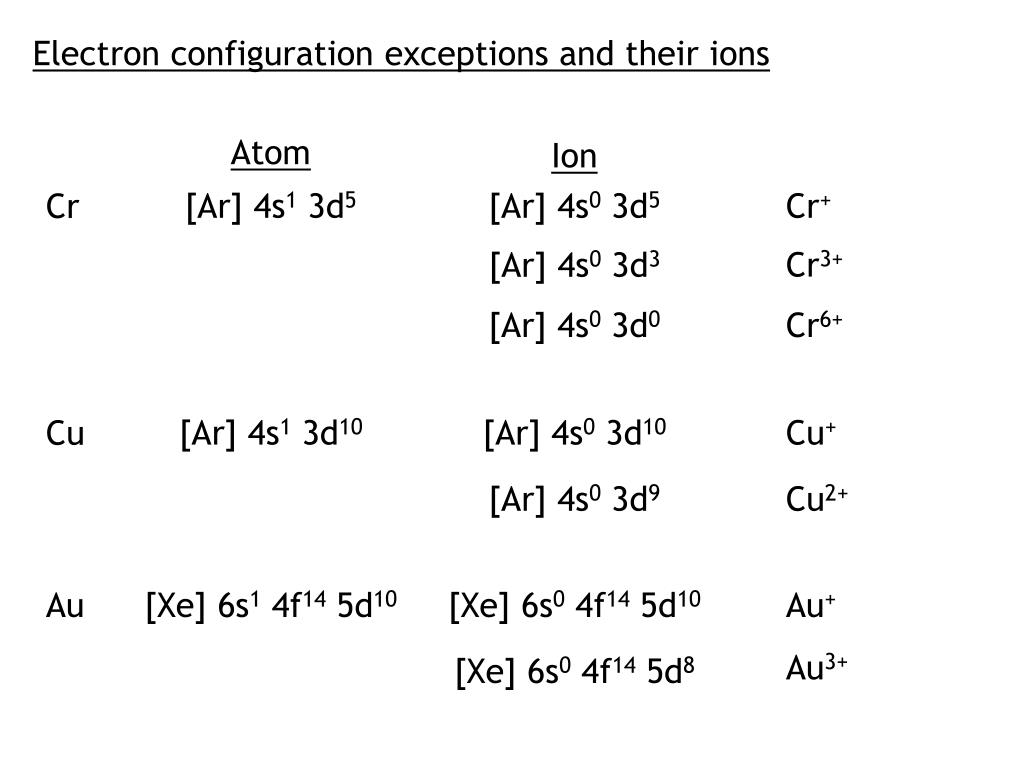
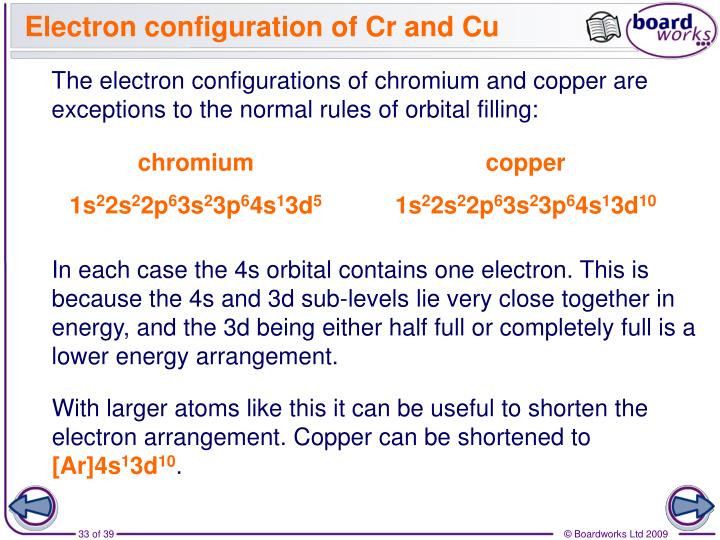
But beyond this point, $3d$ becomes lower in energy than $4s$, so you lose $4s$ electrons first when we're talking about ionization. For reasons that go beyond the scope of this answer, before you hit the transition metal elements ( $Z \le 21$), $3d$ is higher in energy than $4s$, so we fill $4s$ before $3d$. To write the configuration for the Chromium ions, first we need to write the electron configuration for just Chromium (Cr). In the case of chromium, we are dealing with orbitals that are almost in the energy ( $3d$ and $4s$) so you can essentially view this as a special case of Hund's rule that extends to orbitals of nearly the same energy.įor what it's worth, the other notable exception is copper, which adopts a $4s^1$ $3d^9$ configuration over $4s^2$ $3d^8$. Recall that Hund's rule essentially states that you fill each orbital once before going back with the second electron in regards to orbitals of the same energy (in the same subshell). For this reason, chromium adopts a $4s^1$ $3d^5$ configuration, in which each electron occupies its own orbital.
In chromium, having a $4s^2$ $3d^4$ configuration results in electron-electron repulsion due to the two electrons in the $4s$ orbital. Yes I believe that's a typo, it should say "permit." The reason we see these Aufbau's principle exceptions in transition metals is because the $4s$ and $3d$ orbitals are very similar in energy. NOTE: Chromium is an exception to the rules for writing electron configurations Video: Cr, Cr2+, and Cr3+ Electron Configuration Notation Electron Configuration for Cr, Cr2+, and Cr3+ (Exception to Rules) In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital.


 0 kommentar(er)
0 kommentar(er)
